Metallic Corrosion – Intergranular Attack
Background
The effects of residual and applied stresses and corrosive environments in service are closely interrelated. The more highly stressed (higher energy) regions of a metal will become anodic and corrosive cells will be set up due to differences in local stress levels. Cold worked regions, for example tube or sheet bends and cut edges, will be corroded in preference to uniform parts of sections in the same way that grain boundaries are attacked more than grain interiors on the microscopic scale.
Stress Corrosion Cracking (SCC) Defined
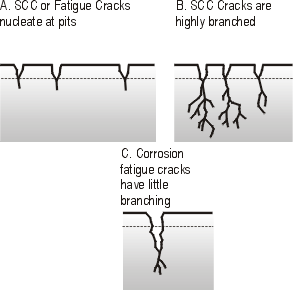
The combined effects of stress and corrosion can result in a special type of failure known as Stress Corrosion Cracking (SCC). This arises under a particular set of circumstances for a given alloy: specific alloy condition plus specific corrosive media and sufficient local tensile stress. Chloride induced cracking of stainless steels, caustic cracking of plain carbon steels and ammonia damage to copper alloys are typical examples of this problem. The mechanism of SCC is shown as a simple representation in Figure 1.
|
|
| Figure 1. Schematic view of Stress Corrosion Cracking (SCC) and corrosion fatigue cracking |
SCC is believed to be nucleated at pitting damage sites and develops under the action of local tensile stresses as a highly branched network of fine cracks. At each crack tip the combined action of the tensile stress and specific ions in the corrosive media cause continual crack propagation with little evidence of local deformation.
In austenitic stainless steels, for example, warm chloride solutions in the presence of residual tensile stress can lead to cracking. SCC tendency is slight in low Ni ferritic and martensitic grades but is severe in the 8-10% Ni austenitic steels. Duplex stainless steels have greater SCC resistance than austenitic since the duplex microstructure helps to inhibit the growth of SCC cracks, which tend to be deflected or arrested at austenite-ferrite interfaces. Maximum resistance is obtained with 50/50 austenite-ferrite microstructures and the dispersion of the two phases should be as fine as possible. The increased interest in the duplex grades stems not only from their high pitting and SCC resistance but also from their higher proof stress level which offers savings in material and weight over austenitic material.
When does SCC occur?
Stress corrosion cracking presents an especially difficult problem, since not only is it highly localised but it can occur in environments that are merely mildly corrosive to the material. The damaging concentration of the harmful ions in that environment may be quite small and difficult to detect and, even in the absence of applied stress, residual stresses in a structure can often be of a sufficiently high level to cause SCC and failure in service.
In a given situation the time of exposure needed to cause SCC failure depends on the stress intensity at any pre-existing or developed crack tip. The concentration of stress at the tip of a sharp crack or flaw can be quantified in terms of the Stress Intensity Factor, K1. It determines the growth rate of SCC cracks for a specific alloy environment combination. Catastrophic failure of a component will occur when this factor reaches a critical value, the Fracture toughness of the material, K1C. This enables the determination of allowable defect size in design to avoid failure under given loading conditions.
In stage 2, the crack growth rate is independent of K1 and depends instead on the corrosive environment and temperature. During stage 2 growth, K1 continues to increase and this leads to the rapid acceleration of the crack in stage 3, and final fast fracture when K1 reaches K1C which is the Fracture Toughness of the material.
The higher the value of K1SCC under given conditions, then the greater is the expected SCC resistance, but some materials do not appear to have a threshold resistance.
<< Home