Introduction Since the discovery of detonation synthesis for ultradispersed diamonds in the 1960s, the full potential of nanodiamonds remains to be explored. Detonation synthesis produces nanosized carbon particles with average diameters of ~5 nm, featuring a diamond core covered by graphitic layers and amorphous carbon. The surface of nanodiamond particles is rich in various functional groups and can be further functionalized. The superior properties of the diamond core combined with a large and chemically tunable surface allow them to be used in a wide variety of ways: initial research performed primarily in the former Soviet Union suggests a broad range of industrial applications. Such uses include chemical and electrochemical co-deposition with metals, ultradispersed diamond-polymer composites, polishing, lubrication, and biomedical applications. Commercially, electroplating and lubricant additives currently show the highest potential. Still there is no accepted opinion concerning the mechanism by which coating properties are improved upon the addition of nanodiamond particles to the deposition bath. Two alternatives are currently under consideration. One explains the improvement by inclusion of nanodiamond into the structure of coatings; the other suggests that nanodiamond improves the deposition conditions resulting in decreased columnar structure and porosity of the coating. Addition of nanodiamond to plated coatings is especially important for Ni-based coatings which are expected to replace the Cr-containing coatings currently in use, which require toxic chemicals for their production. Ni-B coatings have been shown to act particularly well. When coated with Ni-B, residual pores in stainless steel or other metals become filled, which results in improved mechanical properties of the interface. The coatings also decrease corrosion and increase wear resistance compared to the steel substrate. However, when compared to the Cr coatings, Ni-B coatings feature lower mechanical properties and wear resistance, both due to their columnar structure. Previous results have shown that nanodiamond additives can increase hardness of Ni-based coatings by a factor of 2-3. Dispersed nanodiamonds added into the deposition bath have been empirically shown to further increase the tribological properties of Ni-B coatings. But still, the role of nanodiamond in the observed phenomena remains unclear. The aim of this study is to investigate the structure and mechanical properties of Ni-B coatings plated with and without nanodiamond additives to the deposition bath to obtain better insight into the role of nanodiamond in the Ni-B deposition process. Experimental Ni-B coatings were deposited on four steel plates via electroless deposition with bath containing diamond nanoparticles supplied by NanoBlox Inc. (USA) and without diamond additives (Table 1). The structure of diamond nanoparticles which form a colloidal solution in the deposition bath, is described elsewhere. For further studies cross-sectional specimen were cut from plates, mounted in thermal-setting plastic and ground using silicon carbide paper and polished using 0.05μm alumina powder dispersed in a polishing cloth. Table 1. A description of the samples used in this study and their mechanical properties determined by nanoindentation. | | | | | | AP | As-plated without ND** | 120 ± 29* | 7 ± 2 | | AP-ND | As-plated with ND | 164 ± 7 | 9 ± 0.5 | | HT | Sample AP heat treated at 385°C for 90 min | 133 ± 14 | 11 ± 0.7 | | HT-ND | Sample AP-ND heat treated at 385°C for 90 min | 254 ± 6 | 10 ± 1.6 | * - Standard deviation
** - ND stands for nanodiamond Mechanical properties were measured via depth-sensing indentation using a Nano Indenter XP (MTS). All indents were performed using a Berkovich tip to a depth of 2 μm. Hardness and moduli are calculated continuously throughout the loading by using the continuous stiffness measurement (CSM) option for all indents on all four Ni-B samples. Raman spectra were recorded to determine the presence of diamond in the samples. Each sample was studied at multiple points using a Renishaw 1000 Raman Spectrometer with an Ar+ laser with excitation wavelength of 514.5nm. Scanning electron microscopy using an FEI XL30 field emission SEM with an energy-dispersive X-ray spectrometer (EDS) was performed to examine the morphology of the coatings and to view residual indent impressions. A GiegerFlex D/Max-B diffractometer (Rigaku) with CuKα radiation was used to perform XRD studies to determine the phase composition of the samples. Results XRD spectra (Fig. 1) confirmed that as-plated samples AP and AP-ND (ND stands for nanodiamond) are X-ray amorphous. A single broad peak centered close to the position of major diffraction peaks of Ni2B and Ni3B, corresponds to amorphous Ni-B (Fig 1 a,b). XRD also shows that the heat treated samples HT and HT-ND are crystalline demonstrating the narrow peaks of nickel borides (Fig 1 c,d). Furthermore, the XRD patterns suggest that though both samples HT and HT-ND have been crystallized after annealing, HT-ND still contains a significant amount of amorphous material as shown by the halo and much finer grains of nickel borides denoted by broader peaks found in this sample. A higher heat treatment temperature or longer treatment time may be needed to fully crystallize the coatings produced in the presence of nanodiamond. A brief description of composition and FWHM values for the predominant Ni2B peak are summarized in Table 2. While XRD allows us to identify nickel boride phases, it does not provide information on presence of nanodiamond in the samples. An XRD spectrum of the nanodiamond powder shown in Fig. 1 e does not correspond with any of the peaks found in the other samples. This suggests that nanodiamond must be below the XRD detection limit if incorporated. 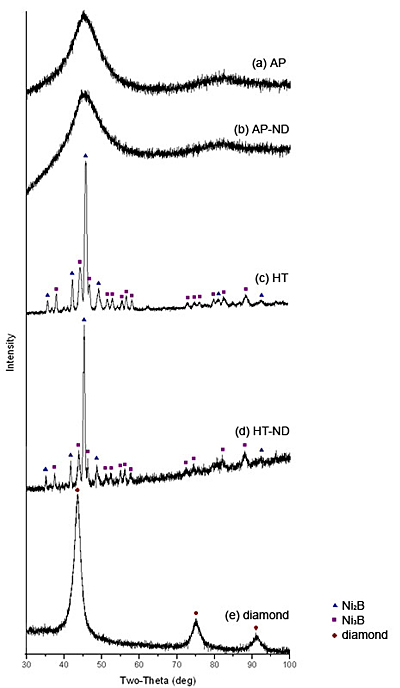
Figure 1. XRD spectra for the samples studied (see Table 1 for description of the samples). Table 2. Composition of the samples as determined by XRD. For the crystalline samples, a calculated FWHM for the (2 1 1) Ni2B peak at 45.9˚ (d = 1.9757 Å) is shown. For the amorphous samples, a calculated FWHM for the amorphous peak is shown. | | | | | AP | amorphous Ni-B | 7.5 | | HT | crystalline Ni2B, and Ni3B | 0.42 | | AP-ND | amorphous Ni-B | 8.4 | | HT-ND | crystalline Ni2B, and Ni3B | 1.27 | The morphology and structure of the coatings was studied by SEM. An SEM image of the cross-section of sample AP-ND clearly shows dendrites which reveal the typical columnar growth mechanism of the Ni-B coatings (Fig 2 a). These columns are believed to be amorphous, as suggested by XRD. When comparing the column size of sample AP and sample AP-ND two differences are observed (Fig 2 b,c). The average width of columns is smaller for samples containing nanodiamonds than without. The average width of dendrites was found to be 0.8 ± 0.1 μm and 0.6 ± 0.05 μm for samples AP and AP-ND, respectively. Furthermore, the addition of nanodiamond leads to straighter columns with fewer branches and arms. 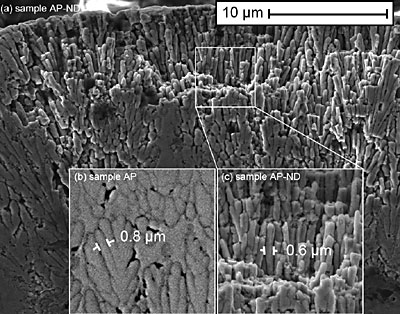
Figure 2. (a) Columnar microstructure of as-plated, AP-ND, sample deposited from the diamond-containing bath. Insets show the microstructure for both samples: (b) the sample deposited without nanodiamond additive, AP, and (c) the sample deposited with nanodiamond, AP-ND. Backscattered SEM images of the samples HT and HT-ND show that the well developed columnar structure significantly decreases as the amorphous material is converted into crystalline grains after heat treatment (Fig. 3). In the case of sample HT, some regions remain columnar or amorphous while other areas, particularly those near the surface transform into crystalline equiaxial grains. This is not the case for sample HT-ND, suggesting that the addition of nanodiamond inhibited grain nucleation and growth. 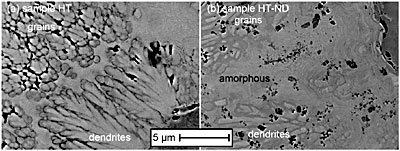
Figure 3. Comparison of the microstructure of the heat treated samples. (a) the sample deposited with no nanodiamond additive, HT, and (b) the sample deposited with nanodiamond, HT-ND. Indentation results for the samples studied are shown in Table 1. Load vs. displacement curves for all samples are shown in Fig. 4. Heat treatment of Ni-B coatings raises hardness. This can be explained by the transformation from the amorphous as-deposited coatings to harder crystalline Ni3B and Ni2B phases as confirmed by XRD (Fig. 1). The addition of nanodiamond also raises hardness by a factor similar to that achieved by annealing and causes a small increase in modulus. Heat treatment of the samples deposited with nanodiamond additives to the bath does not further increase the hardness but does increase the modulus by a factor of two. These measured properties are in agreement with the load vs. displacement curves (Fig. 4) which show an increase in the required load with heat treatment or the addition of nanodiamond. Therefore, such coatings are suitable for raising modulus and hardness or raising hardness without requiring heat treatment. Combination of heat treatment and addition of nanodiamond did not lead to a further increase in hardness as compared to sample HT or sample AP-ND. This can be explained by the fact that the microstructure of sample HT-ND still contained amorphous material (Fig. 1 d). If the sample could be processed in such a way to form small crystalline grains in the presence of nanodiamonds, additional hardening may be achievable. |
<< Home