| When an internal combustion engine burns fuel, heat is created at temperatures as high as 4000°F (2200°C). This heat must be removed by some form of cooling. The two most common ways to dissipate heat are by air cooling or liquid cooling. This paper discusses only the liquid cooling systems used in most modern engines. A liquid cooling system contains the following components; radiator, fan, thermostat, water pump, engine water jacket and the cooling liquid. This paper will concentrate on the liquidcontained inside the cooling system. The analysis of used coolant samples has been a successful technique for scientific preventive maintenance. It is applicable to any closed loop cooling system, but is applied primarily to diesel and gasoline engines because they are the most likely component to suffer from a poorly operating cooling system. Overheating causes oil deterioration, oxidation, reduced lubricity and damage to all oil wetted components. The longevity of liquid cooled transmission and hydraulic system components are also dependent on a properly operating cooling system. A properly maintained cooling system not only prevents overheating but also maintains a constant engine temperature. Improperly maintained engine temperatures can result in the type of problems shown in Table 1. Table 1. Problems due to improper engine temperatures | | | | Pre-ignition | Unnecessary wear | | Detonation knock | Poor fuel economy | | Lubrication failure | Accumulation of water and sludge | | Burnt pistons and valves | | Why Coolant Analysis Most people don’t give much thought to the condition of their engine coolant system until it is too late. More than 40% of all diesel engine maintenance problems can be attributed to poor cooling system maintenance. Poorly operating cooling systems cause the engine to run hotter which in turn causes the lubricant to oxidize and loose it’s lubricity thus causing abnormal wear in all oil wetted areas. The following is a list of reason why to do coolant analysis. · Protect against gel formation · Protect against corrosion and rust · Protect against over/under concentration of SCA’s · Extend drain intervals · Protect your engine · Environmental/disposal concerns Another factor that recently gained world-wide attention is the impact of used coolant disposal on the environment. Ethylene glycol is extremely hazardous if ingested by humans or animals. Because of this, most large users of coolants operate the coolants longer in order to reduce the need for disposal. Others have started recycling and reconditioning the used coolants. Because of this, the need for coolant analysis has increased dramatically over the past few years. Disposal of used coolants can be difficult and expensive and must be done in accordance with local, state or federal laws. The following is the Cummins Engine Company recommended cooling system maintenance intervals: · Replace coolant filter at every oil change. · Top off the cooling system at filter changes. · Test/replenish SCA package at filter change. · Test the coolant twice a year. · Replace coolant every two years or 240,000 miles (6,000 hours). The following is the Caterpillar Inc. (CAT SOS coolant analysis) recommended cooling system maintenance intervals: · Every 250 hours check glycol level, freeze and boil protection, SCA concentration, pH, and conductivity. · Every 1000 hours or a minimum of twice a year check the same as above, plus identify metal corrosion, contaminant levels and built-up impurities. Coolant Analysis To be effective, a used coolant analysis program should determine both the coolant condition and the presence of any contaminants or debris. The coolant fluid can be used as a diagnostic medium as the coolant carries not only heat away from the engine parts but also carries fine debris from the interior surfaces of the cooling system. Analysis of the wear debris can provide important information about the condition of the internal parts of the cooling system. However, the condition of the coolant itself is important to know. Does the coolant meet specification? Is the SCA package correct? Is the coolant contaminated with solids, metal particulate or chemical degradation products? In a modern condition monitoring program based on coolant analysis, a coolant sample is taken from a piece of equipment at periodic sampling intervals. Good and consistent sampling practice is extremely important. Samples should be taken from the radiator or block drain, never from the surge tank or coolant recovery bottle. The sample is sent to the laboratory for analysis. Based on the analysis, a diagnostic report is made and a recommendation is sent to the personnel responsible for the equipment. The report may show that everything is normal, warn of a possible problem or make a specific maintenance recommendation. The entire process, from sample taking to the diagnostic report, should take less than 24 hours. A sample report is shown in Fig. 1. 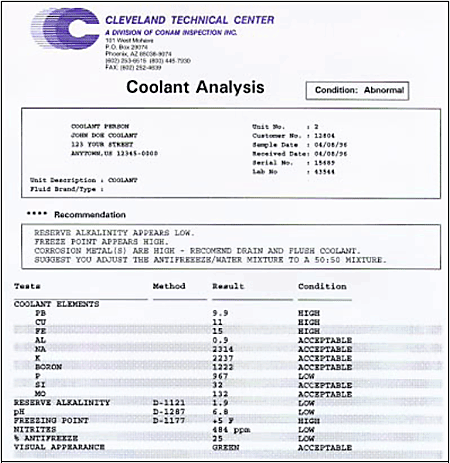
Figure 1. Sample coolant analysis report In a modern coolant analysis program, the data generated and collected by the laboratory is also used to provide periodic maintenance summaries. These reports can be statistical in nature and provide an insight to management personnel on the effectiveness of the program, efficiency of the maintenance department, repair status of equipment, recurring problems, and even information on the performance of coolants. Condition monitoring by coolant analysis can be broken down into two main categories: · Debris Monitoring · Coolant Condition Monitoring Debris Monitoring Debris monitoring spectrochemically measures the trace elements carried away from the cooling system by the coolant. Coolant Condition Monitoring Coolant condition monitoring determines if the coolant itself is fit for service based on physical and chemical tests. These two techniques, when combined with statistical trending and data-based management, provide a complete program of condition monitoring by coolant analysis. Debris Monitoring for Coolants Debris monitoring pertains primarily to the detection of metallic wear particles, corrosion products, degradation products and contaminants. Spectroscopy is the most widely applied technique for debris monitoring. Commercial labs in the USA have been using either ICP or AA spectrometers for coolant analysis. Table 2 lists elements routinely detected and quantified for coolant analysis. Table 2. Elements routinely detected and quantified coolant analysis | | | | | Iron | Silicon | Potassium | | Zinc | Magnesium | Silicon | | Lead | Calcium | Boron | | Copper | | Sodium | | Aluminium | | Molybdenum | | Magnesium | | Phosphorous | Table 3 lists the typical elements which are routinely analyzed and provides examples to their origin in a diesel engine cooling system. Table 3. Sources of various elements in used coolants | | | | | Iron (Fe) - Liners, water pump, cylinder block, cylinder head | Silicon (Spectro Incorporated) - Dirt | Potassium (K) - Buffer | | Zinc (Zn) - Brass from components | Magnesium (Mg) - Hard water scaling problem | Silicon (Si) -Anti-foaming agent, anti-corrosion for aluminum | | Lead (Pb) - Solder in radiator, oil cooler, after cooler, heater core | Calcium (Ca)- Hard water scaling problem | Boron (B) - pH buffer, anti corrosion for ferrous metals | | Copper (Cu) - Radiator, oil cooler, after collar, heater core | | Molybdenum (Mo) - Anti-cavitation, silicate | | Aluminum (Al) - Radiator tanks, coolant elbows, piping, spacer plates, thermostat housing | | Phosphorus (P) - pH buffer, anti corrosion for ferrous metals | | Magnesium (Mg) - Cast alloys | | | The RDE/AES technique has been in use for over 50 years for the analysis of a variety of samples. Water samples were routinely analyzed by RDE spectrometers. Low limits of detection were achieved by first concentrating the sample by evaporating most of the water by putting the water sample in an oven. With the introduction of the ICP/AES technique in the late 1970’s, water samples were no longer run on RDE spectrometers because of the significantly lower limits of detection as well as superior precision offered by ICP spectrometers. Concentration of water samples by evaporation was no longer necessary, at least not for routine samples, because the limits of detection were so very much lower. For lubricating oil and fuel analysis, the RDE technique continued to be a preferred method due to its simplicity of operation and reliability. Sample introduction is simple. Both AA and ICP spectrometers require that the oil sample first be diluted, usually with kerosene, so that the sample can be nebulized to form an aerosol. The RDE technique also has the ability to more efficiently analyze the larger particulate in the used sample. RDE spectrometers lend themselves to on-site analysis in less than optimum working environments whereas AA or ICP spectrometers required a laboratory environment as well as more highly skilled personnel for their successful operation. Recently, several RDE spectrometers for commercial customers were calibrated with coolant standards so that these spectrometers could be used not only for oil analysis but also for coolant analysis. The limits of detection (LOD) that can be achieved on a RDE instrument are not as low as can be achieved using an inductively coupled plasma (ICP) spectrometer, but for coolant analysis low limits of detection are not very important since the results are rounded off to the nearest ppm for reporting purposes and sub-ppm levels are of no practical consequence. Table 4 compares the LOD’s for ICP and RDE spectrometers and shows that the RDE spectrometers are appropriate for coolant samples. Table 4. Limits of detection | | | | | | | Na | 0.04 | 0.20 | 0.015 | 0.03 | | Mo | 0.46 | 0.08 | 0.005 | 0.37 | | Mg | 0.01 | 0.20 | 0.0002 | 0.01 | | P | 3.00 | 0.50 | 0.008 | 3.20 | | B | 0.07 | 0.01 | 0.002 | 0.13 | | Ca | 0.03 | 0.03 | 0.0001 | 0.03 | | Cu | 0.04 | 0.02 | 0.002 | 0.06 | | Al | 0.35 | 0.02 | 0.001 | 0.43 | | Pb | 0.80 | 0.10 | 0.02 | 0.75 | | Fe | 0.23 | 0.02 | 0.002 | 0.21 | | Si | 0.20 | 0.10 | 0.006 | 0.33 | | Zn | 0.10 | 0.02 | 0.002 | 0.18 | It may be of interest to note that the LOD’s for oil are not substantially different than those obtained for water (or for water/glycol mixtures). LOD’s are not by any means the only measure of spectrometric performance. In the case of coolant analysis the purpose is to detect abnormal circumstances. LOD’s have little to do with the quality of coolant analysis furthermore, real measurements are many times LOD so that LOD’s much less than 1 ppm are not important. The ICP technique measures only the most finely divided material, i.e., the material cannot be in the form of large particles. This is because the sample introduction system of an ICP spectrometer includes a spray chamber, the purpose of which is to remove the largest aerosol droplets generated by the nebulizer. The size of the aerosol droplets which reach the plasma torch where emission takes place are on the order of 1 or 2 mm. The RDE spectrometer, on the other hand, is able to detect somewhat larger particles, up to and beyond 10 mm in size. Table 5 and Table 6 compare results obtained with an ICP spectrometer to results obtained on an RDE spectrometer on used coolant samples. Table 5 is a comparison of used coolant samples that were relatively clean, (no particulate could be visibly detected). Table 6 is a comparison of used coolant samples where particulate could be visibly detected. The data clearly show that if particles are present the results for the wear metals and contaminants are substantially higher when the sample is run on the RDE spectrometer. It may be concluded from this data that neither instrument is entirely quantitative for these samples because there will typically be some fraction of larger particles that is not completely measured by either an ICP or RDE spectrometer. Nevertheless, the purpose of the analysis is served by indicating which coolant systems are in distress. It may be argued that since the RDE spectrometer is more responsive to large particles it is more capable of indicating abnormal coolant conditions than an ICP spectrometer. Table 5. ICP/RDE Comparison of “clean” coolant samples | | | | | | | | | | | | | | | RDE | 1.1 | 0.4 | 0 | 0 | 0 | 0.5 | 0 | 47.6 | 18.6 | 590 | 389 | 2069 | | ICP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 41 | 19 | 406 | 248 | 1078 | | RDE | 14.8 | 6 | 7.8 | 8.7 | 2 | 0 | 0 | 37.6 | 0 | 122 | 263 | 1546 | | ICP | 6.7 | 0 | 3.8 | 3.2 | 0 | 0 | 0 | 26 | 0 | 90.2 | 217 | 914 | | RDE | 1.3 | 0.4 | 0 | 0 | 0 | 0.1 | 0 | 34.8 | 0.2 | 511 | 649 | 2541 | | ICP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 28 | 0 | 315 | 421 | 1309 | | RDE | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 3.2 | 13.5 | 77.5 | 615 | 2409 | | ICP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.7 | 18 | 60 | 480 | 1283 | | RDE | 0.4 | 0.7 | 5.7 | 7.1 | 1.3 | 0.5 | 0.8 | 40 | 58.9 | 984 | 635 | 2835 | | ICP | 0 | 0 | 2.7 | 2.8 | 0 | 0 | 0 | 26 | 44 | 585 | 345 | 1385 | | RDE | 0.8 | 0.7 | 1 | 0 | 0 | 0 | 0 | 3.7 | 0 | 445 | 325 | 1364 | | ICP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.6 | 0 | 335 | 213 | 852 | | RDE | 0.3 | 0.9 | 0.6 | 6 | 1.9 | 0.4 | 12.1 | 61 | 1.2 | 122 | 840 | 5972 | | ICP | 0 | 0 | 0 | 2.6 | 0 | 0 | 3.6 | 43 | 0 | 74 | 515 | 2650 | Table 6. ICP/RDE Comparison of coolant samples where particles were detected | | | | | | | | | | | | | | | RDE | 0.2 | 39 | 0 | 39 | 8.7 | 0.9 | 0 | 53.6 | 0 | 1677 | 630 | 5440 | | ICP | 0 | 12 | 0 | 17 | 2.5 | 0 | 0 | 34 | 0 | 1163 | 321 | 2733 | | RDE | 11 | 23 | 60 | 2.8 | 4.3 | 1.3 | 0 | 34.2 | 2.5 | 61.8 | 806 | 3365 | | ICP | 6 | 5.6 | 37 | 0 | 0 | 0 | 0 | 23 | 4 | 53.7 | 672 | 2134 | | RDE | 0.5 | 47 | 0 | 0 | 0 | 0.2 | 0 | 59 | 23.3 | 583 | 1415 | 6471 | | ICP | 0 | 8.7 | 0 | 0 | 0 | 0 | 0 | 30 | 15 | 271 | 748 | 2552 | | RDE | 2 | 1 | 1 | 4.9 | 2.8 | 1.3 | 5.8 | 42.4 | 68.5 | 326 | 1088 | 5013 | | ICP | 0 | 0 | 0 | 2.2 | 0 | 0 | 0 | 29 | 58 | 203 | 678 | 2668 | | RDE | 0.9 | 16 | 75 | 25 | 40 | 0.9 | 4.2 | 12.4 | 0.2 | 12.1 | 1368 | 5118 | | ICP | 0 | 2.3 | 28 | 7.5 | 19 | 0 | 0 | 5.2 | 0 | 12.5 | 870 | 2495 | | RDE | 1.1 | 1.7 | 24 | 2.5 | 2.5 | 0.4 | 0.1 | 24.5 | 66.3 | 709 | 385 | 1652 | | ICP | 0 | 0 | 7.5 | 0 | 0 | 0 | 0 | 19 | 61 | 522 | 232 | 959 | | RDE | 9.4 | 12 | 114 | 49 | 6.9 | 10 | 66.4 | 29.4 | 0.6 | 948 | 1424 | 6567 | | ICP | 0 | 0 | 25 | 8.9 | 0 | 2.2 | 12 | 12 | 0 | 561 | 746 | 2904 | | RDE | 0 | 28 | 130 | 70 | 14 | 3.7 | 1.2 | 39 | 7 | 327 | 322 | 1671 | | ICP | 0 | 0 | 47 | 18 | 6.7 | 0 | 0 | 32 | 8.7 | 222 | 211 | 939 | | RDE | 5.7 | 44 | 1.5 | 3.4 | 28 | 27 | 269 | 150 | 260 | 2110 | 751 | 3585 | | ICP | 0 | 12 | 0 | 0 | 7.6 | 7.4 | 44 | 83 | 193 | 1315 | 334 | 1564 | Coolant Physical Property Monitoring The second part of an effective coolant analysis program is coolant condition monitoring. Through periodic sampling of the coolant, the laboratory can determine the effectiveness and remaining life of the coolant based on additive degradation and contamination analysis. ASTM (American Society for Testing and Materials) tests are mostly written for quality control and quality assurance requirements of new and sometimes used coolants. Therefore, ASTM procedures are often modified to reduce analysis times in the interest of economics. The number and type of tests that are performed on a used coolant sample vary. Table 7 summarizes the physical property tests performed by the typical used coolant analysis laboratory. Physical Property Tests for Used Coolant Analysis Table 7. Typical physical property tests for used coolant analysis | pH, ASTM 1287 | | Reserve Alkalinity, ASTM D1121 | | Percent EG/PG | | Freeze/Boiling Point | | Nitrite, ppm NO2 | | SCA Levels | | Total Dissolved Solids (TDS) | | Appearance | pH pH measures the acidity or alkalinity of the used coolant sample. This can be measured by performing ASTM D1287, which is very precise. The use of an inexpensive pH meter can provide quick and accurate results in a non-laboratory environment. Most pH meters have the capability to measure the conductivity of the coolant which also can be used to determine the percentage of TDS. Most major engine manufacturers recommend coolant pH levels between 8.5 to 10.5. If pH levels fall below 8.0 rapid nitrite depletion will occur. Coolant pH levels above 11.5 will corrode aluminum and promote scaling. Reserve Alkalinity Reserve alkalinity measures the amount of alkaline inhibitors present in the used coolant. This gives an indication of the coolant’s ability to provide corrosion protection. This test can be performed by ASTM D1121. If the buffering agents are not at correct levels, corrosion and rapid additive depletion will occur due to a reduction in pH values. The result will be cylinder liner pitting. Percent Antifreeze EG/PG This test uses a refractometer to quantify the amount of EG/PG in a coolant sample. Most major engine manufacturers recommend coolants composed of 50/50 water/glycol solution to provide satisfactory freeze and boil point protection. An operating range of 40 to 60 % antifreeze is acceptable, however, the use of antifreeze in concentrations over 65% may cause SCA drop-out, water pump seal damage and engine overheating. Freeze/Boiling Point Once the percent of EG or PG is determined, the freeze and boiling point can be calculated by using charts provided by the antifreeze manufacture. Freeze point can also be measured by performing ASTM D1177. If the percent antifreeze is not known, an inexpensive hydrometer can be used to measure the density of the coolant and then calculate the freeze point. The amount of freeze protection required should be based on the lowest expected temperature in your region. Nitrites/SCA Package Analysis The analysis of the primary corrosion inhibitor, nitrite, can be performed by various tests. The most accurate method is by ion chromatography. For the most accurate results this test must be performed in a laboratory. The second method is the colormetric analysis with nitrite test sticks. This is a very quick and easy way to measure the concentration of NO2. Fleetguard offers a test stick that will measure nitrites (NO2), molybdates (MoO4) and freeze point protection colormetricly on the same test stick. As with all elements contained in the SCA package, the concentrations must remain within 10% of the new coolant SCA additive level. Total Dissolved Solids (TDS) This is a measurement of the dissolved solids in the coolant. The dissolved solids are composed of the basic inhibitor chemicals, silicates, active SCAs, spent SCAs, contaminants and water hardness compounds. The higher the dissolved solids the higher the conductance. The percentage TDS can be quantified with a conductivity meter. This meter will also be capable of measuring the pH of the coolant. Cummins recommends a maximum of 5% TDS, higher levels may cause water pumps seal failure. Appearance This quick and easy test records the overall condition, color and visible contamination of the coolant. Color is important because most manufacturers identify coolants by color. It is important to notify maintenance personnel if oil or large particles are present in the coolant sample. Conclusion RDE spectrometers, when appropriately calibrated, may be used as part of a condition monitoring program based on coolant analysis. The same instrument as used for used oil analysis can be modified and calibrated to also effectively analyze coolants. The added capability provides the laboratory with a supplementary tool to increase its capabilities and effectiveness. |